test
Test
I guess the picture is you with el pyjama, but I can't see it, sorry.
Don't worry about it, it was just a picture of my dog to see if we were able to send images attached to the questions:)
Ok, so now we want to see your dog!
jajajajjajajaja (test)
test
Hello Giovanna,
I have a question regarding the synthesis of the heme group.
I was wondering if you could give me more details about the insertion of the Fe by the ferrochelatase. I don't understand how does the oxidation of two of the nitrogens allow the insertion of the Fe. I'd be greatful if you could give me more details about the bond that is established between the Fe and the four nitrogens.
Thanks beforehand.
Guille.
Dear Guillermo,
finally some questions!
The bond establishing between iron (in this case Fe2+) and the four nitrogen atoms is the so-called coordinate-covalent bonds, that are covalent bonds, where the electron pair that is shared between 2 atoms is provided only by 1 atom. In the case of heme, nitrogen atoms can provide the electron pairs that are accomodated in the empty orbitals of Fe2+.
Fe2+ can form up to six coordination bonds, so that is why the 5th ligand is usually an amino acid of the protein (His for example) and the 6th position can be occupied by another amino acid ligand or by oxygen or by other electron donating ligands.
Does it answer your question?
Dear Giovanna,
Thanks for giving me more information about it.
There's still one thing that I don't fully understand. If the Nitrogens atoms are providing the electrons for the coordinated bond, wouldn't it make more sense if they were reduced instead of oxidized in the previous reaction?
Thanks beforehand.
The oxidation reaction is very important because it removes 6 electrons and protons and introduces 3 new double bonds, allowing the porphyrin ring to become a highly conjugated system (with a typical color). In a conjugated system, electrons are delocalized and yoy can write resonance forms.
Nitrogens have already electron pairs for the coordination bond with iron, they do not need to be reduced.
In the next reaction catalyzed by ferrochelatase, there is the removal of two protons from -NH.
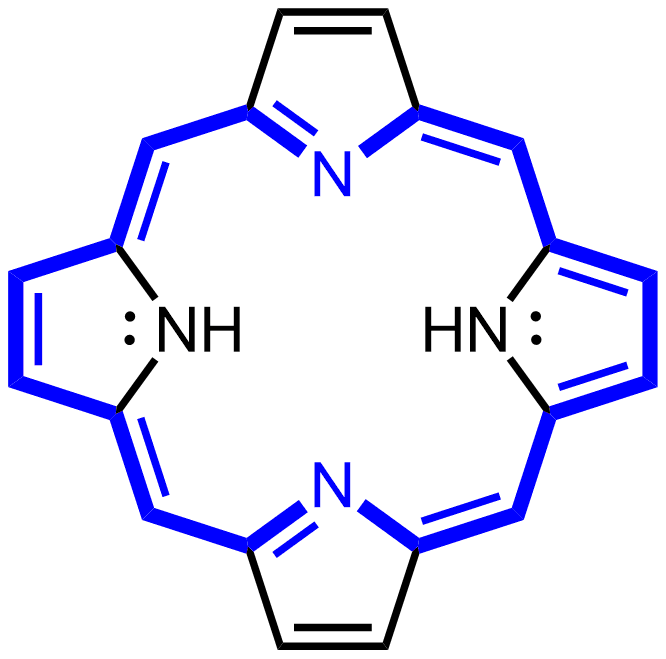
Oh alright, that makes way more sense. Now I understand the oxidation reaction.
Thanks for everything!
Good afternoon,
I have a question regarding the oral part of the exam, do we have to memorize PLP and tetrahydrobiopterin formulas?
Thank you,
Michela
Yes, if a reaction is supposed to be memorised, also the coenzymes, cofactors required should be.
Thank you very much. I have another question, this time on a specific topic:
In lesson 7 I don't undestand why when Ca level are low (I imagine in the blood) the hormone 1,25(OH)D3 stimulates osteoblast to take up Ca and not to release. Viceversa I see why it stimulates Ca absorption in the diet.
Thank you,
Michela
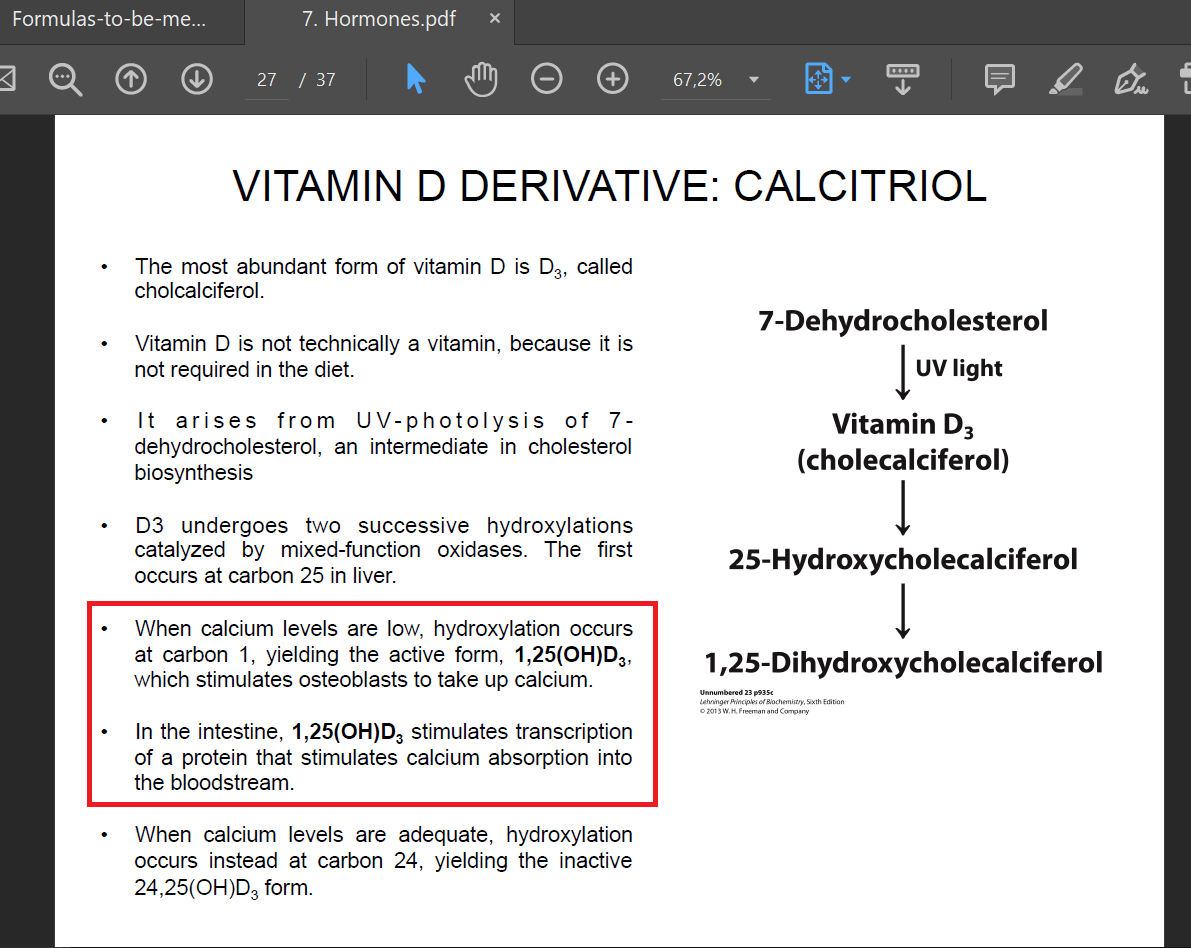
The homeostasis of calcium is regulated by different hormones and we didn't go into detail of PHT and calcitonin.
In general, vitamin D stimulates osteoblasts to take up calcium and bone mineralization in order not to loose calcium from bone, that acts also as a reservoir of calcium itself.
The hormone stimulating the release of calcium from bones is mainly the parathyroid hormone (PHT, we didn't talk about it).
To increase calcium blood levels, other actions are promoted by vitamin D (intestine and kidney) so that levels increase without affecting bone. Indeed, vitamin D deficiency stimulate the release of calcium from bones whereas optimal levels promote calcium absorption.
Thank you, now it's very clear!
Best regards,
Michela
