Knocking-down and knocking-out genes
(Restore this version)
Modified: 17 April 2019, 6:59 PM User: Giulia Pirro →
Back to index
Tentative subjects:
1. Transient siRNA/LNA-mediated knock-down
2. Stable shRNA knock-down
3. K.O. in cultured cells, alternatives
(Giulia Pirro)
The RNAi (RNA interference) is a class of dsRNA that allows the silencing of a target gene.
The shRNA (short hairpin RNA or small hairpin RNA) is an artificial construct which is able to bind in a specific way the mRNA coding for the known gene.
The sequence of the shRNA is formed by a sense strand, an antisense strand (able to anneal with the previous) and a loop region in between.

Usually, shRNA is the key in order to obtain a knock-down. If the gene targeted for knock-down has a known biological or physiological function, would be easier and be extremely useful in testing the efficacy of an shRNA.
STEPS IN ORDER TO SILENCE A TARGET GENE:
- First of all the plasmids, which contains the shRNA construct, should be transfected in the cells. There are several ways in order to obtain the transfection, one of the most common is the lipid-base transfection.
- If these transfections occur, each cell is able to express the shRNA.
- The hairpin loop, which strongly characterize the molecule, has to be removed by the DICER. After this processing the shRNA will become siRNA.
- SiRNA is able to bind the protein complex RISC (RNA-Induced Silencing Complex) and from dsRNA (double-stranded RNA) it becomes single strand RNA.
- The complex formed by both RISC and the single stranded RNA, is able to bind the mRNA target, which is complementary to the shRNA construct, and cleaves it.

The crucial point between these steps is the stable transfection. The plasmid should contain both the shRNA construct but also a resistance gene. In this way is easier both the selection and the isolation of successfully transfected cell.
In order to better understand if the actually phenotype is the result of this treatment (and exclude any unspecific effect) is necessary perform few proves:
1. REDUNDANCY: producing more than one shRNA construct. These constructs share the same translation, but not the same nucleotides sequence. If these constructs are specific for a target mRNA the results will be the same.
2. SCRAMBLED: produce the shRNA construct which doesn’t show a perfect matching. Without the match should be express the target gene. Silencing doesn’t occur. Usually this mechanism is used like a negative control.
This kind of technique is largely use in biotechnology field in order to obtain a specific pattern of expression and moreover to investigate in a specific link between expression and phenotype.
(Ilaria Ghia)
A knockout cell line (constitutive or conventional KO) is a model in which a target gene is permanently inactivated and thus is not able to produce the protein it encodes for.
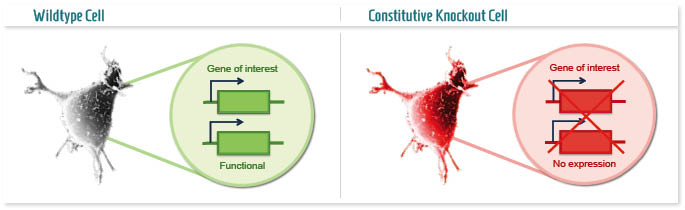
The applications of this technology are many, and can be distinguished on the type of research it is used for:
- Academic research: study of the main functions of a gene and/or protein, LOF (Loss-of-function) studies, in vitro recapitulation of human diseases;
- Bio-pharmaceutical research: in vitro target validation, drug study and screening through mimicry of human diseases,study of safety, off-target activity and specificity of a drug.
As every technique, knockout cell lines show some strength and limitations. Three main advantages are:
- Total absence of protein, excluding all the possible isoforms;
- Since each cell line is matched with an isogenic (=genetically identical) control cell line, it is a reliable methodology;
- Feasible in every genetic background or cell type.
By contrast, three weaknesses are:
- Genetic compensation: the function of the KO protein can be supplied by the action of other related proteins;
- Non-specific phenotype: the editing of a gene can sometimes affect the neighboring genes too;
- Not very suitable for highly polyploid cell lines.
Nevertheless, these limitations can be minimized with bioinformatic, genetic and bibliographic analyses. Moreover, an alternative is represented by functional knockouts, in which the use of CRISPR/Cas9 or other genome editing methods allows the introduction of a point mutation to produce an inactive protein.
The generation of KO cell lines requires several steps, summarized by the following image.

Clone screening is needed to select the clones that correctly integrated the plasmid, which come in the end in a small number, reducing the efficiency of the process. An alternative is to use the newest technologies in genome editing, such as TALEN and ZNF (besides the most diffused CRISPR/Cas9, which is discussed in the Genome Editing section of this Wiki: http://cmb.i-learn.unito.it/mod/wiki/view.php?pageid=114).
